Why mass spectrometry of virus-like particles (VLP) and adeno-associated viruses (AAV)?
VLP’s are becoming more and more important as biologics due to their ability to dock onto cell surfaces and release their payload into the cells. They are used for vaccinations, gene therapies and other applications. However, VLP or AAV characterzation is not so easy because of the size of these particles as well as their heterogeneity.
Common ways of analysis are the characterization of their individual components and their size. But a number of questions are not answered by this:
- is the composition correct?
- is the correct payload present?
- is the correct amount of payload present?
The direct determination of the mass of the intact AAV or VLP particle by native MS can adress these questions whereas e.g. SEC-MALS only provides size information. The size change of the particle however is only in the range of a few percent whereas the mass changes dramatically upon inclusion of the RNA payload.
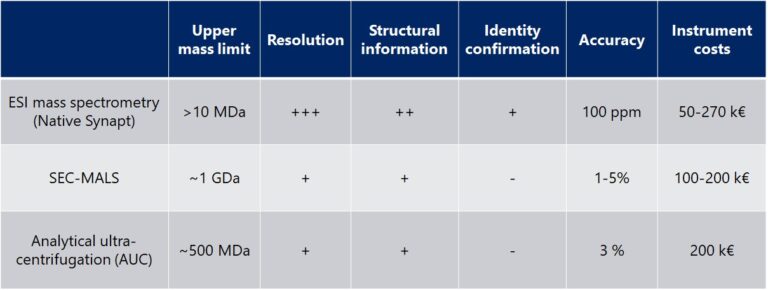
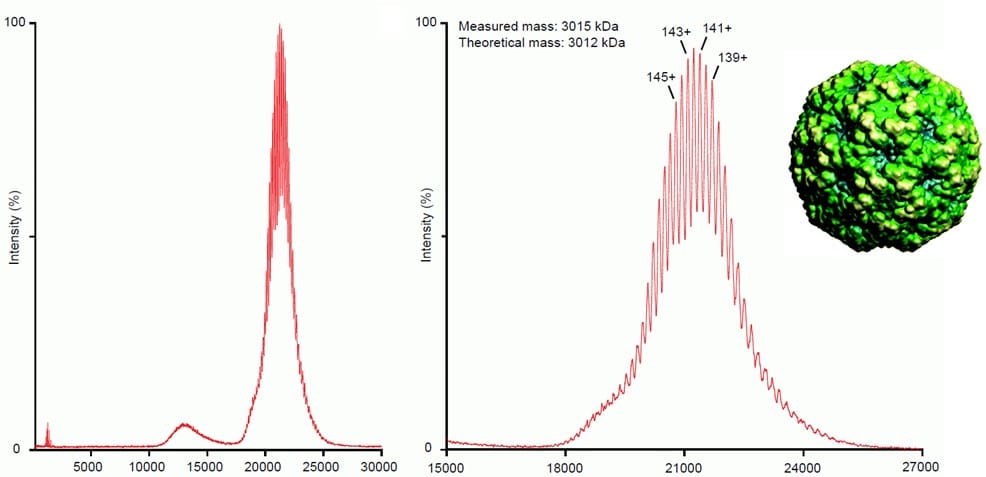
How can native mass spectrometry help in the analysis of virus capsids/AAV’s?
Native MS has been successfully applied to characterize AAV’s and other VLP’s (see literature list below) by mass spectrometry. Native MS allows for directly determining the ration between unloaded and loaded particles, purity of the product, correct composition (otherwise the mass would not match) and even for decomposing the particle for in-depth composition characterization.

Enabling an existing Waters Synapt system with native MS capability to characterize VLP’s is cheaper than you think and will provide you with a great ROI!
VLP and AAV capsid characterzation by native mass spectrometry – Literature:
Learn more about the application of MS Vision’s dedicated high mass QTOF and Synapt systems for VLP and AAV characterization by mass spectrometry:
- G.K. Shoemaker et al., “Norwalk Virus Assembly and Stability Monitored by Mass Spectrometry”, Mol. Cell. Prot. (2010), p 1742-51, DOI: 10.1074/mcp.M900620-MCP200
- J. Snijder et al., “Studying 18 MDa Virus Assemblies with Native Mass Spectrometry”, Angew. Comm. (2013)52:4020-23, DOI: 10.1002/anie.201210197
- R. Pogan et al., “Norovirus-like VP1 particles exhibit isolate dependent stability profiles”, J. Phys.: Condensed Matter (2018)30:064006, DOI: 10.1088/1361-648X/aaa43b
- V.U. Weiss et al., “Virus-like particle size and molecular weight/mass determination applying gas-phase electrophoresis (native nES GEMMA)”, Anal. Bioanal. Chem. (2019)411:5951-62, DOI: 10.1007/s00216-019-01998-6
- S. Zoratto et al., “Molecular weight determination of adeno-associate virus serotype 8 virus-like particle either carrying or lacking genome via native nES gas-phase electrophoretic molecular mobility analysis and nESI QRTOF mass spectrometry”, J. Mass Spectrom. (2021)56:e4786, DOI: 10.1002/jms.4786
