Overcoming the limitations of standard MS instrumentation in the analysis of large protein structures has been the focus of our efforts over the past decade. By working together closely with leading academic groups, we learnt how to get the best out of the mass spectrometer. The result is an instrument which allows researchers to unleash the full capacity of mass spectrometry for the analysis of intact proteins and complexes by native electrospray mass spectrometry:
- Antibody analysis
- Antigen-Antibody interaction studies, stoichiometry, affinity studies
- Antibody PTM analysis
- Protein Complexes, protein-protein interactions
The interest in MS of large biomolecules and biomolecular complexes has significantly increased in the past years as more and more pharmaceuticals are either new biological entities (NBE) or biosimilars/biobetters. However, their characterization is critical to understand their activity. Complex formation can lead to inactivity or even toxic effectsand posttranslational modifications affect efficacy as well as half-life in the body. Due to the heterogeneity and size of many biologics molecules, the analytical requirements differ significantly in comparison to small molecules or peptides.



Turning the QExactive into a biopharma analysis workhorse
With long-lasting experience in modifying Waters Synapt systems to the NativeSynapt, optimizing the ThermoFisher QExactive platform for biopharmaceutical application was a logical next step.
A couple of years ago, ThermoFisher Scientific had launched a Biopharma upgrade option, which increases the mass range of the QExactive. Yet this option has three significant limitations:
- First, the detection mass range is only extended from 6.000 to 8.000 m/z, which for native MS applications of monoclonal antibodies is just sufficient. 90% of our customers would like to have more mass range available.
- Second, the selection mass range of the quadrupole remains at 2.500 m/z. As a consequence, a signal of a monoclonal antibody can hardly be selected as the vast majority of the charge envelope is above 2.500 m/z.
- Third, HCD fragmentation at the pressures used in QExactive is not optimal for very large molecules as they can dissipate the vibrational activation due to their size. Top-down or middle-down approaches are hardly used despite their significant advantages regarding sequence coverage.
What makes the NativeQE so special for biopharma?
To enable user in biopharma applications, MS Vision now offers the NativeQE as complete instrument or upgrade (both currently being based on QE plus systems).
The NativeQE has a couple of modifications which dramatically improve the usability of the system for the characterization of monoclonal antibodies and other biologics.
Extended mass range
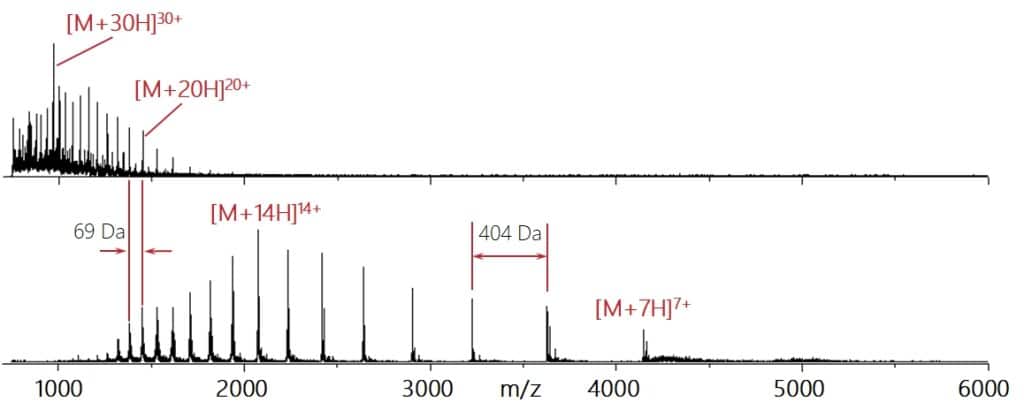
First, we increased the detection mass range to 10.000 m/z to fully cover the mass range relevant for monoclonal antibodies under native conditions as well as many non-covalent protein complexes. The spectrum below was acquired from a 10 μM concanavalin A solution in 100 mM ammonium acetate with nESI source and activated charge reduction.
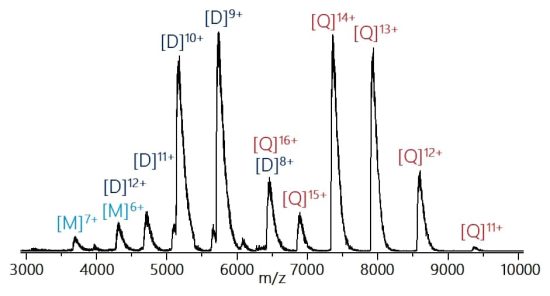
Higher quadrupole isolation limit
But the detection mass range alone is not enough when you want to characterize biologics. With complex mixtures like glycosylated antibodies or ADC’s you want to characterize each isoform individually to find out where the glycosylation or drug is localized. This requires the isolation of specific isoforms which can be performed by selection based on their specific mass-to-charge ratio. As the original QExactive only offers selection up to m/z 2.500, MS Vision worked on a solution for this challenge as well.
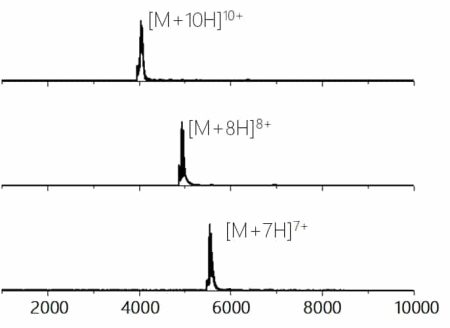
We transferred our 20 years of high mass knowledge on Waters mass spectrometry technology to increase the quadrupole selection mass range from 2.500 m/z to 10.000 m/z. This allows to isolate specific charge states or isoforms of your antibody or NBE for further subsequent characterization.
The picture on the left shows the isolation of different charge states of ovalbumin (~40 kDa) produced using nESI of a 5 μM solution in 100 mM ammonium acetate.
A critical question in particular for native mass spectrometry is whether the isolation is soft enough to preserve non-covalent interactions. In the example on the right, Myoglobin was analyzed using native mass spectrometry, generating signals of the holo-Myoglobin. The 5+ charge state at m/z 3.514 was isolated and showed no signs of fragmentation. Only by activating an additional CID potential of 85V the loss of the heme group was observed resulting in the observation of holo- and apo-Myoglobin signals.
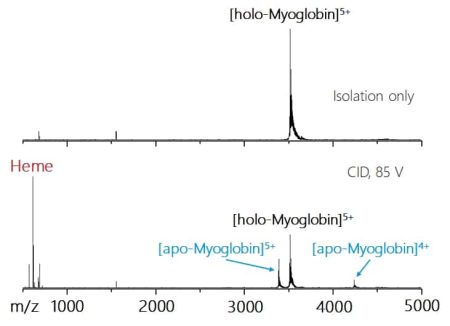
Improved CID fragmentation
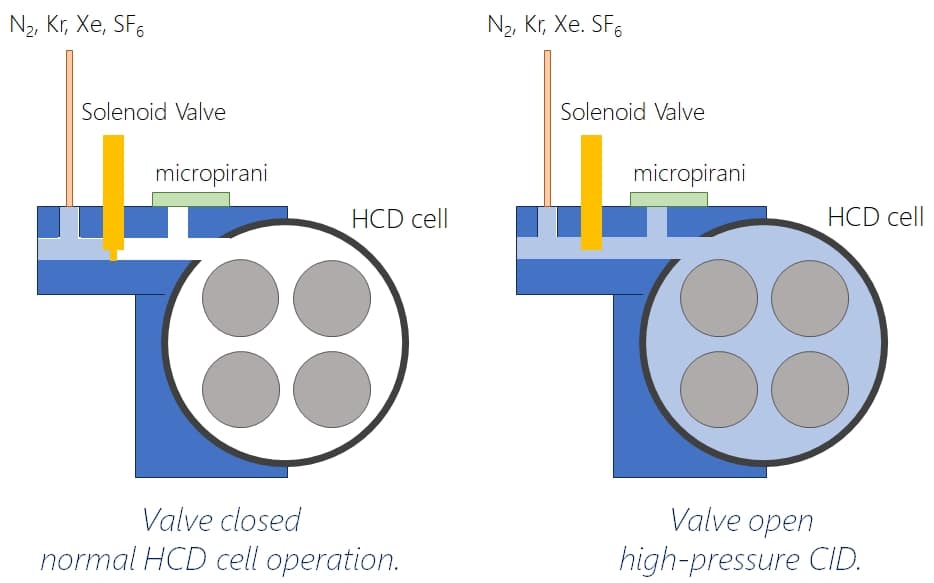
Large proteins are intriniscally difficult to fragment by collision-induced dissociations as they can dissipate the vibrational energy more easily. To achieve a more efficient fragmentation on larger molecules, one can either increase the collision energy (which is sometimes difficult for trapped ions as they might fly out of the trap due to the high speed), use collision gases with larger molecular mass or increase the pressure (=amount of collisions) in the collision cell. In QExactive systems, there is a limitation of the overall pressure in the system as this influences the mean free path length in the Orbitrap detector and high pressure deteriorates resolution. To achieve good fragmentation, MS Vision has now added a pulsed gas valve to the HCD cell which temporarily increases pressure and also allows to use more heavy gases for improved fragmentation.
Optional charge reduction
Furthermore we added a charge reduction device as well as provisions for UVPD fragmentation to our upgrade package. The charge reduction shifts the mass-to-charge ratio to higher values and, more imporantly, increases the distance between adjacent charge states. This improves isolation of highly charges species as well as deconvolution of overlapping charge states of highly heterogeneous molecules. Also, an inherent drawback of native MS is that the sensitivity is lower than for conventional ESI from acidic solutions. The charge reduction now allows to achieve the sensitivity of the conventional ESI combined with the spacing and resolution of charge states of the native MS!
As an option for the future, we also provide a new backend of the HCD collision cell which allows for coupling of lasers for ultraviolett photodissociation (UVPD) in the future.
No compromise
The NativeQE from MS Vision is an upgrade for the Thermo Fisher QExcative platform with focus on QExactive plus. The upgrade is available as field upgrade or as complete system. It includes higher detection AND higher selection mass range, improved gas handling for more efficient fragmentation of large ions without compromising Orbitrap detection performance as well as an optional charge reduction to improve resolution on complex mixures by increasing the difference between charge states.
As the Netherlands are famous for creating space since 1600 AD, we thought it’s time to create space again – for your ions and between the QExactive plus/HF and the QExcative UHMR:
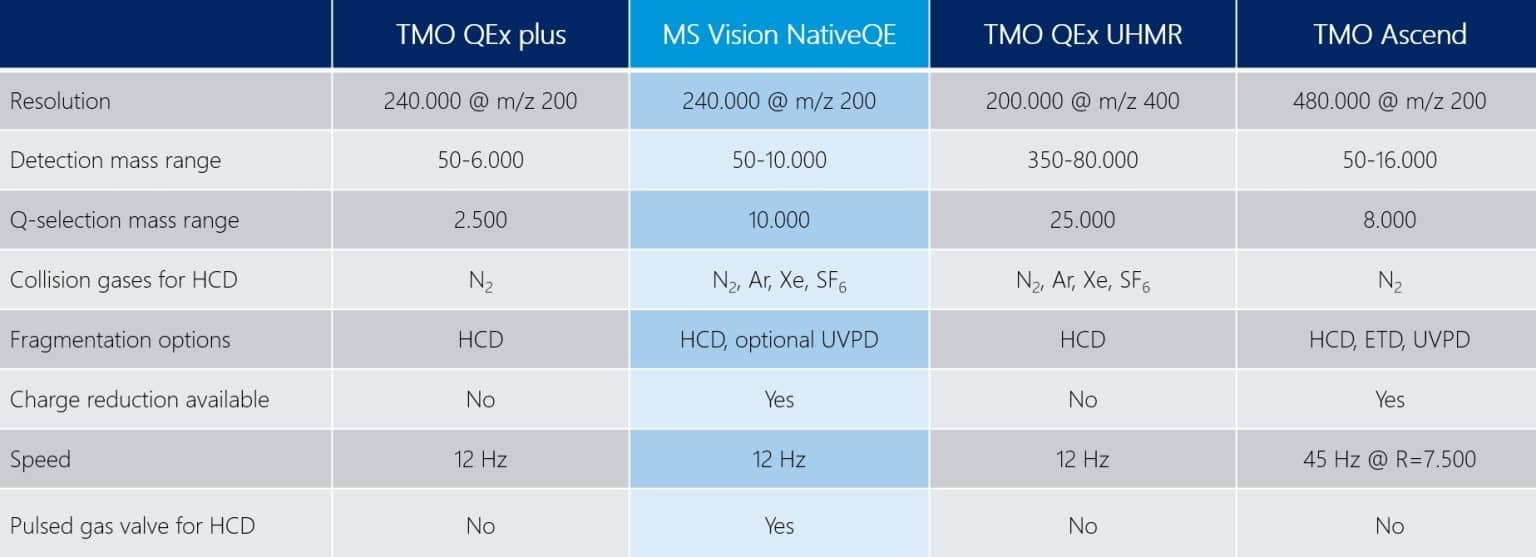
Upgrade or full instrument - your choice and flexibility
Whether you want to have your own QExactive upgraded or need a complete instrument, MS Vision has the solution. We offer full support by our highly skilled engineers so you can concentrate on running your samples.
Native Mass Spectrometry is in demand and the ThermoFisher QExactive type instruments can be upgraded by MS Vision for Native MS applications.
The system will be completely refurbished and is better than new!
A new PC to drive the entire system and to help you with your analysis using the complete suite of Thermo software will be supplied with the instrument. The system will be installed in your laboratory and will meet specifications before it will be transferred to you. After this we will provide a training to enable you to properly operate the system and get the full functionality.
The warranty is 12 months on the entire system, not only on the upgrade parts! Following the warranty service contracts from a Bronze type, PM only, to full cover Platinum type are available.
Contact us to discuss your demands and we will be happy to send you a detailed quotation
